
Calculate the molality.Įxample #7: Calcuate the molality when 75.0 grams of MgCl 2 is dissolved in 500.0 g of solvent. Sometimes, a book will write out the word "molal," as in 0.500-molal.Įxample #5: Calculate the molality of 25.0 grams of KBr dissolved in 750.0 mL pure water.Įxample #6: 80.0 grams of glucose (C 6H 12O 6, mol. In the above problem, 58.44 grams/mol is the molar mass of NaCl. Step Two: divide moles by kg of solvent to get molality. The solution to this problem involves two steps. What would be the molality of the solution? After all, chemists use balances to weigh things and balances give grams, NOT moles.Įxample #4: Suppose you had 58.44 grams of NaCl and you dissolved it in exactly 2.00 kg of pure water (the solvent). Now, let's change from using moles to grams.
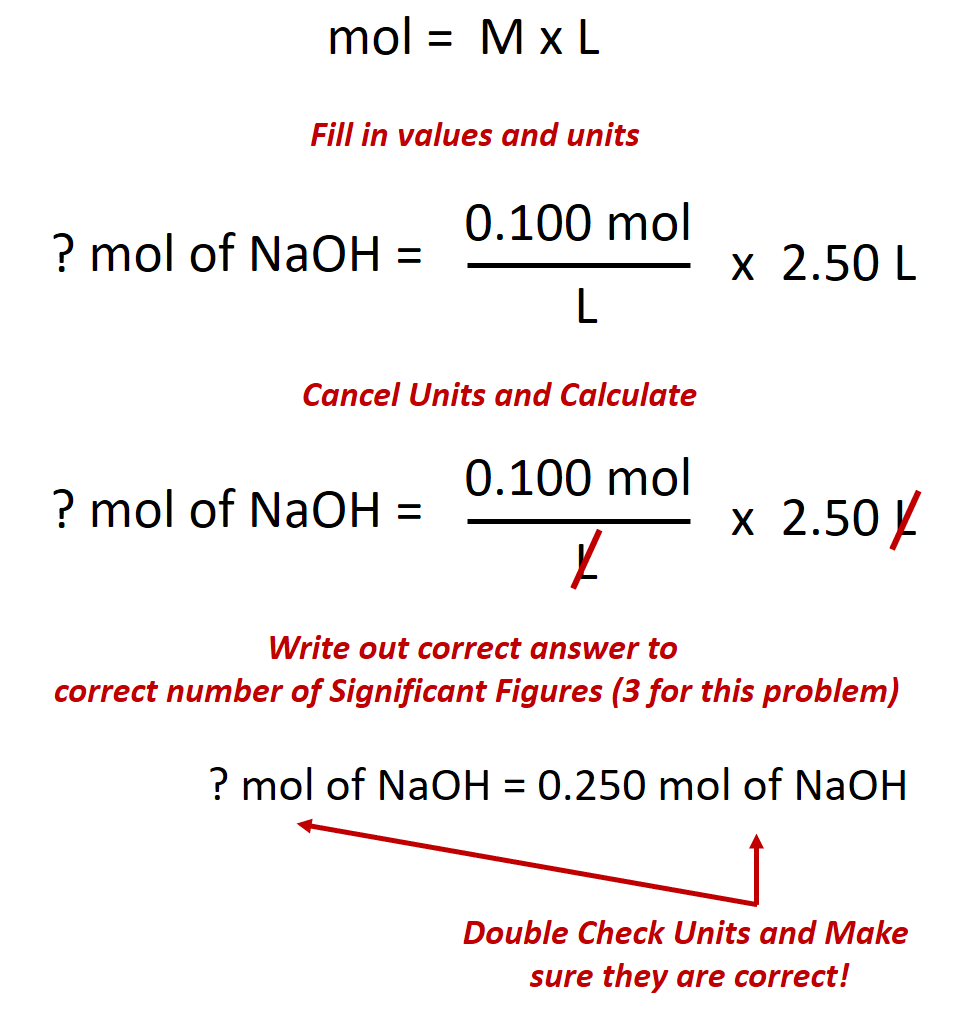
One mole of anything contains 6.022 x 10 23 units.Įxample #3: What is the molality when 0.750 mol is dissolved in 2.50 L of solvent? It doesn't matter if it is sucrose, sodium chloride or any other substance. The molarity would be the same no matter what the substance. Notice that no mention of a specific substance is mentioned at all. It is not the actual unit.Įxample #2: Suppose you had 2.00 moles of solute dissolved into 1.00 L of solvent. The m is a symbol that stands for mol/kg. When you say it out loud, say this: "one point oh oh molal." You don't have to say the dash.Īnd never forget this: replace the m with mol/kg when you do calculations. (A lower-case m is also used for meter, but the context should be clear that m means molality.) Maybe including the dash would be wise if there might be a potential misunderstanding Having said that, however, be aware that often m is used for mass, so be careful. However, if you write 1.00 m for the answer, without the italics, then that usually is correct because the context calls for a molality. Some textbooks also put in a dash, like this: 1.00- m. It is a lower-case m and is often in italics, m.


Neither cancels.Ī symbol for mol/kg is often used. Notice that both the units of mol and kg remain. What would be the molality of this solution? Notice that my one liter of water weighs 1000 grams (density of water = 1.00 g / mL and 1000 mL of water in a liter). We then made sure everything was well-mixed. We keep adding water, dissolving and stirring until all the solid was gone. This is probably easiest to explain with examples.Įxample #1: Suppose we had 1.00 mole of sucrose (it's about 342.3 grams) and proceeded to mix it into exactly 1.00 liter water. The molality of a solution is calculated by taking the moles of solute and dividing by the kilograms of solvent. The unit mol/kg requires that molar mass be expressed in kg/mol, instead of the usual g/mol or kg/kmol.As is clear from its name, molality involves moles. A solution of concentration 1 mol/kg is also sometimes denoted as 1 molal. This contrasts with the definition of molarity which is based on a specified volume of solution.Ī commonly used unit for molality in chemistry is mol/ kg. Molality is a measure of the number of moles of solute in a solution corresponding to 1 kg or 1000 g of solvent.


 0 kommentar(er)
0 kommentar(er)
